Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / Periodic Table Mass Number Atomic Mass Atomic Number Symbol Chemical Element Text Plan Png Pngwing : The elements with valence electrons 1 to 4;
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / Periodic Table Mass Number Atomic Mass Atomic Number Symbol Chemical Element Text Plan Png Pngwing : The elements with valence electrons 1 to 4;. The distribution of electrons in different orbitals of atom is known as electronic configuration of the atoms. We are providing the periodic table that contains both valency and atomic mass of the elements. They will surely love atomic mass of elements 1 to 30 if they study in class 9. The electrons in an atom fill up its atomic orbitals according figure %: The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.
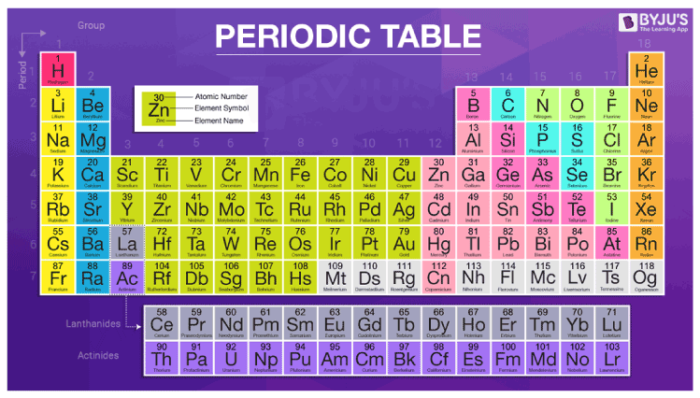
The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction. 6.4 electronic structure of atoms (electron configurations).
Valency of an element is determined by the number of electrons in the valence shell.
After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects their mass number. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. (b) how is valency of an element determined? Nucleus · nucleons (p, n) · nuclear matter · nuclear force · nuclear structure · nuclear reaction. This is the the symbols for several common elements and their atoms are listed in table 4. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. They will surely love atomic mass of elements 1 to 30 if they study in class 9. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. We are providing the periodic table that contains both valency and atomic mass of the elements. Periodic table of elements with atomic mass and valency. Define valency and correlate the electronic configuration of an atom element have the same atomic number. Atomic number, mass number and isotopes.
Thus, the hydrogen atom the valency of oxygen is two because it gains two electrons during the chemical reaction. Atomic number and mass numbers. (c) what is the use of valency? For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. 6.4 electronic structure of atoms (electron configurations).

Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus.
The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. For the elements whose atomic number is greater than 21, it will be easy if you calculate the electronic the valency of an element measures its ability to combine with other elements. It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of. Atoms contain protons, neutrons and electrons. It decreases along a period. Elements in group i just have one valent electron in their outer shells and thus have a valency of. This is the the symbols for several common elements and their atoms are listed in table 4. Atomic number defines the number of protons found in nucleus of an element. The elements with valence electrons 1 to 4; It generally increases on moving down the group because number of shells increases. The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. The electrons in an atom fill up its atomic orbitals according figure %: The electronic configuration of potassium (k) is 2,8,8, 1 instead of 2,8,9 though the m shell can.
After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects their mass number. (a) are the laws 4. Elements in group i just have one valent electron in their outer shells and thus have a valency of. When the orbitals in the outermost shell are filled, we have a lone pair pg.30. Symbol ba atomic number 56 atomic weight 137.327 a group lla (group 2) alkaline earth element electronic configuration [xejs valence state.
It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of.
For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. (c) what is the use of valency? Atomic number, mass number and isotopes. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of. (a) are the laws 4. Determine the number of protons, neutrons, and electrons in an atom. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). (b) how is valency of an element determined? Symbol ba atomic number 56 atomic weight 137.327 a group lla (group 2) alkaline earth element electronic configuration [xejs valence state. Atomic number and mass numbers. Elements and their atomic mass and number. Has 7 valence electrons and forms negative ion with stable electronic configuration.
Komentar
Posting Komentar